Hi Anisha,
First of all, it is important to realize that electrons do not circle around a nucleus as we often see in pictures or as we previously thought.
They do not circle at a definite path and don't have a set position but they exist in a sort of cloud. However, they keep on moving.
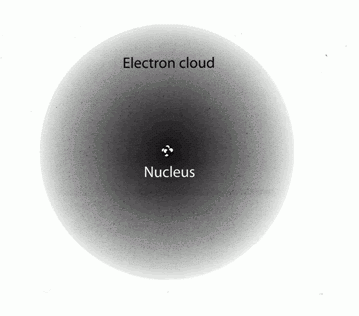
To do so, they need energy otherwise they would collapse. Common knowledge suggests us that to move we need to spend energy. However, the first law of Newtonian mechanics says:
"The velocity of a body remains constant unless the body is acted upon by an external force."
This means that there is no need to spend energy continuously unless there are external forces that are acting on a set body. The electrons found in a nucleus are in a quantized energy level and this can change only if there is some external interaction. This quantized energy is called quanta, introduced by Niels Bohr. This is a discrete energy states in which electrons can persist stably, meaning that there's a minimum state below which the electron cannot fall. The point that quantum mechanics makes on this, is that there is no energy coming in or out the nucleus-electron system unless there is an external interaction. In effect, we can only know what the electron is doing if we interact with it, that's why there is no set definition. So, if there is no interaction we can only guess its distribution and use probability to figure it out.
The conclusion is: an electron has no need for a continuous source of energy to keep on moving as its movement is balanced by the stable energy level (quanta) that it is found in the nucleus. Thus it keep on moving. This is what I got from my research anyway, hope this clarifies your doubts!

