Francium
Francium
But shouldn't Francium be the smallest? It's below Cesium
According to my knowledge, all the alkaline earth elements have the smallest Ionization energy. These include Li, Na and K.
As indicated in the image attached, the lowest Ionization energy's are those of the Alkali Earth metals of Group IA of the Periodic Table of the Elements. Please also consult the URL provided for an explanation of Ionization energies of the Periodic Table.
images.google.com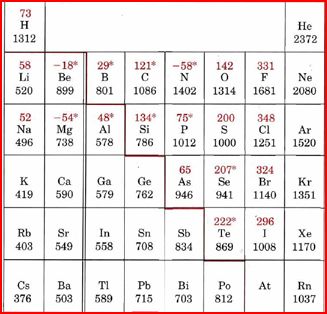
images.google.com

According to Mastering Chemistry it's Cs, don't know why though according to the trends.
In the periodic table the the ionization energy increases from left to right and decreases while moving down. According to this cesium
is found of the bottom left corner of periodic table so it has the smallest ionization energy.
is found of the bottom left corner of periodic table so it has the smallest ionization energy.