Tim Cook answered
CoCl4 2- is the result of adding hydrochloric acid to a water-based solution of cobalt chloride, and is also known as cobalt tetrachloride.
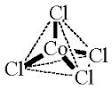
Uses for Cobalt Chloride (CoCl2)
Outside the laboratory, the main use of cobalt chloride is as a humidity indicator, making it useful for equipment used to forecast and measure weather conditions. Cobalt chloride changes color when humidity increases, starting as a blue substance, then changing from purple, then pink when very humid.
This is because the chloride molecules have to 'make room' for the absorbed water around the central cobalt molecule.
Here's an experiment showing how cobalt chloride can be used to demonstrate Le Chatelier's Principle: