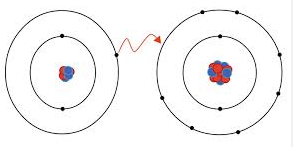
NaCl---Common table salt, made of sodium and chlorine.
Sodium, Na, is the cation and donates one electron to chlorine and is written------ Na^+
Chlorine is the anion and accepts one electron from sodium into its valance shell and is written-----Cl^-
Both are neutral as a solid, but in solution both are charged ions and will conduct electricity.