Platinum is a transition metal with a variable valency.
Platinum has 6 stable isotopes and an oxidation transition of 4, (2).
Platinum is a transition metal with a variable valency.
Platinum has 6 stable isotopes and an oxidation transition of 4, (2).
Platinum can have 1-3 valence electrons, as it is in the transition metals group.
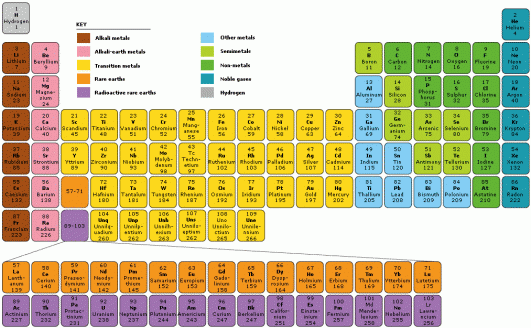
The first column/group, known as the alkali metals group, will always have 1 valence electron, and will be attracted to elements that have 7 valence electrons. When they group together, they complete the 8 max valence electrons.
The second column/group, known as the alkali-earth metals group, will always have 2 valence electrons, and be attracted to elements that have 6 valence electrons.
Elements in the third group will always have 3 valence electrons, thereby attracting to elements with 5 valence electrons.
The fourth group will have elements with 4 valence electrons, fifth group 5 valence electrons, sixth group 6 valence electrons, seventh group 7 valence electrons, and eighth group 8 valence electrons.
Didn't find the answer you were looking for?