As more and more heat transfer into the liquid, the kinetic energy of the liquid atoms starts to increase, breaking free from that loose bond and creating vapors, where all sorts of atoms from that liquid now roam free within the atmosphere.
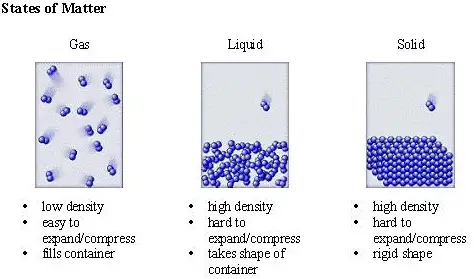
As more and more heat transfer into the liquid, the kinetic energy of the liquid atoms starts to increase, breaking free from that loose bond and creating vapors, where all sorts of atoms from that liquid now roam free within the atmosphere.

This is so because when a liquid reaches a set temperature its molecules are susceptible to evaporation. Thanks to the four states of matter and years of experiments.
Didn't find the answer you were looking for?