Building a model of an atom of an element such as Lithium is a fun way to learn, and to teach others, about chemistry and the Periodic Table. But forget about building a model to scale, unless you've a couple of miles to play with!
How to Make Your Atom
You need 10 small circular items of 3 different colors to represent the neutrons, protons and electrons (If you make 3 of the circular items smaller than the other 7, so much the better.
An adhesive is required to join the protons and neutrons together, and some sort of thin metal strong enough to 'skewer' whatever you're using for the electrons.
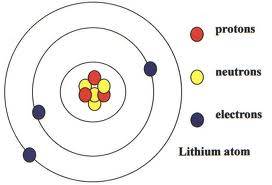
Take 7 of the circular items (say, plastic balls), 4 of one color (say, red) and 3 of another (for example, yellow). Use glue, or something thing and sharp, such as cocktail sticks, and press the 7 yellow and red balls as tightly together as possible until all are stuck to each other without any chance of one ball falling off. This is your Lithium nucleus.
The three other balls, all of which should also be the same color, (let's say green) will form the orbiting electrons.
To give a sense of the circular paths around the nucleus, cut three differing lengths of wire (craft wire is best). Skewer a green ball onto the end of each, and bend each wire into a rough circular shape, with the other end of the wire piercing the other side of the green ball.
A dab of glue might help keep the ends of the wire in place within the ball.
Place the three wire-skewered balls into an arrangement around the 'nucleus', the shortest wire nearest, the longest wire furthest. And there you have your model Lithium atom, looking something like in the diagram.
This YouTube user had a go at building a model Lithium atom. Watch to see how she got on: