I would use rubber bands to illustrate energy levels, pennies to represent the orbiting electrons, and then red and blue marbles for the protons in red and neutrons in blue consistent with the image of oxygen attached. 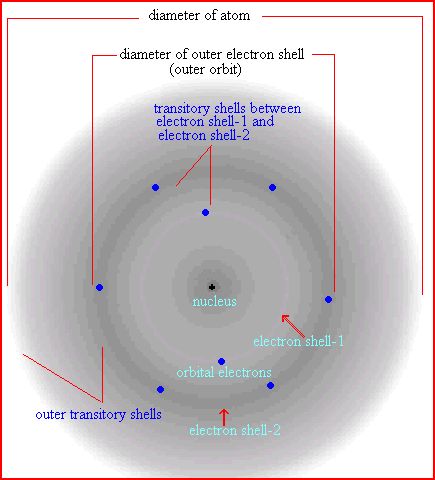


Didn't find the answer you were looking for?