Magnesium is the element of Group II of the periodic table. The atomic number of magnesium is 12 and its mass number is 24.31. You can check the uploaded picture and electronic configuration is explained below:
There are three shells in magnesium atom and there are two electrons in the first shell, eight in the second shell and two in the third shell. There are total 12 electrons in magnesium atom and 2 electrons are present in the outermost shell of magnesium. For more details: Magnesium atom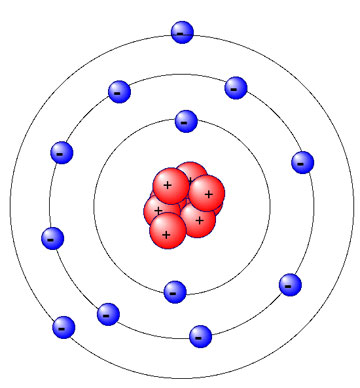
There are three shells in magnesium atom and there are two electrons in the first shell, eight in the second shell and two in the third shell. There are total 12 electrons in magnesium atom and 2 electrons are present in the outermost shell of magnesium. For more details: Magnesium atom
